Doctors in the UK, the US, and beyond are prescribing more generic drugs than ever - but that doesn’t mean they all trust them. Even though generics make up 90.1% of all prescriptions filled in the US, only about 32.7% of physicians consistently choose them as first-line treatment. Why the gap? It’s not about cost. It’s about what doctors believe - and what they’ve been told.
Many doctors still doubt generic effectiveness
A 2017 survey of 134 Greek physicians found that more than 25% believed generic drugs were less effective than brand-name versions. That number hasn’t dropped much since. Even today, nearly 27.3% of doctors still question whether generics truly match the therapeutic performance of branded drugs. This isn’t just a gut feeling - it’s rooted in real experiences. Some doctors report seeing patients react differently after switching from a brand to a generic. A common example? Levothyroxine. Patients on this thyroid medication often report changes in energy, weight, or heart rhythm after a switch. While the FDA says generics are bioequivalent - meaning they deliver the same active ingredient within an 80-125% range - some clinicians argue that even small variations matter for drugs with a narrow therapeutic index. Reddit threads from practicing doctors in 2023 show that 62.3% of respondents have seen at least one adverse event they linked to switching generics in these sensitive cases.Age, experience, and specialty shape opinions
Not all doctors think the same way. Older physicians, especially those with over 10 years of experience, are more likely to stick with brand-name drugs. Male doctors also show stronger skepticism than female counterparts - a surprising twist, since earlier studies assumed women were more cautious. Specialists like cardiologists and neurologists are far more hesitant than general practitioners. Why? They treat conditions where precision matters: seizures, heart rhythm disorders, thyroid imbalances. A 2% difference in drug absorption might not mean much for an antibiotic, but it can trigger a seizure or a stroke in someone on warfarin or phenytoin.Education gaps are the real problem
Here’s the kicker: most doctors think they understand how generics work. But when tested, only 43.7% of primary care physicians in one Oxford study could correctly explain bioequivalence standards. Meanwhile, 86.1% said they lacked sufficient continuing education on the topic. Many learned about generics in medical school - if they learned anything at all. Only 38.7% of U.S. medical schools include structured training on generic drug equivalence, according to the AAMC. This knowledge gap leads to bad habits. Doctors who don’t fully grasp the science often avoid generics not because they’re dangerous - but because they’re unsure. They don’t want to risk a patient’s health over a $10 savings. And when they do prescribe generics, they often don’t explain why. That silence feeds patient fear.Patients learn from doctors - and trust fades fast
Here’s a vicious cycle: if a doctor hesitates to prescribe a generic, the patient picks up on it. They hear the hesitation in the tone, see the pause before signing the script. Then they Google it. They read horror stories online. They start refusing the cheaper option. The CDC found that 41.7% of rural patients stopped taking their meds altogether after being switched to a generic - not because it made them sick, but because they didn’t trust their doctor’s judgment anymore. And here’s the worst part: when patients lose trust in one decision, they start doubting everything. A patient who refuses a generic might also skip a vaccine, delay a screening, or ignore lifestyle advice. The mistrust doesn’t stop at pills. It spreads.
Pharmacists know more - and trust generics more
The contrast between doctors and pharmacists is stark. While 28.7% of physicians doubt therapeutic equivalence, only 22.1% of pharmacists do. Pharmacists see the data daily - batch numbers, manufacturing records, bioequivalence reports. They’re the ones who fill the script, answer questions at the counter, and watch what happens when patients take the drug. They know most generics work just fine. Yet pharmacists can’t prescribe. They can’t override a doctor’s hesitation. All they can do is offer information - and sometimes, that’s not enough if the doctor doesn’t believe it themselves.What changes minds? Real evidence - not lectures
A 2017 study in Greece tested a simple fix: give doctors real-world data. A 90-minute workshop showed them outcomes from actual patients who switched to generics. No theory. No slides about FDA rules. Just charts showing blood pressure levels, thyroid tests, seizure frequency - before and after the switch. The result? 37.2% improvement in positive attitudes. And six months later, those doctors were prescribing generics 22.5% more often. The biggest jump? Among physicians with 5-10 years of experience. Not the rookies. Not the veterans. The ones still learning. Peer influence worked better than any expert lecture. Doctors who saw a colleague successfully switch their patients to generics were 43.2% more likely to follow suit than those who got training from outsiders.Regulations vary - and so does trust
In Europe, the European Medicines Agency requires generics to stay within a tighter range: 90-111% bioequivalence. In the U.S., it’s 80-125%. That difference isn’t just technical - it affects perception. Germany has an 18.4% higher rate of generic use than the U.S., and part of that comes from tighter standards and more public confidence. The FDA’s 2023 GDUFA III rules now require more post-market data on generics. Early results from Johns Hopkins show that when doctors get access to real patient outcomes - not just lab reports - their prescribing habits shift. In a pilot program, generic use for newly approved drugs jumped by 28.6% when providers saw actual clinical results.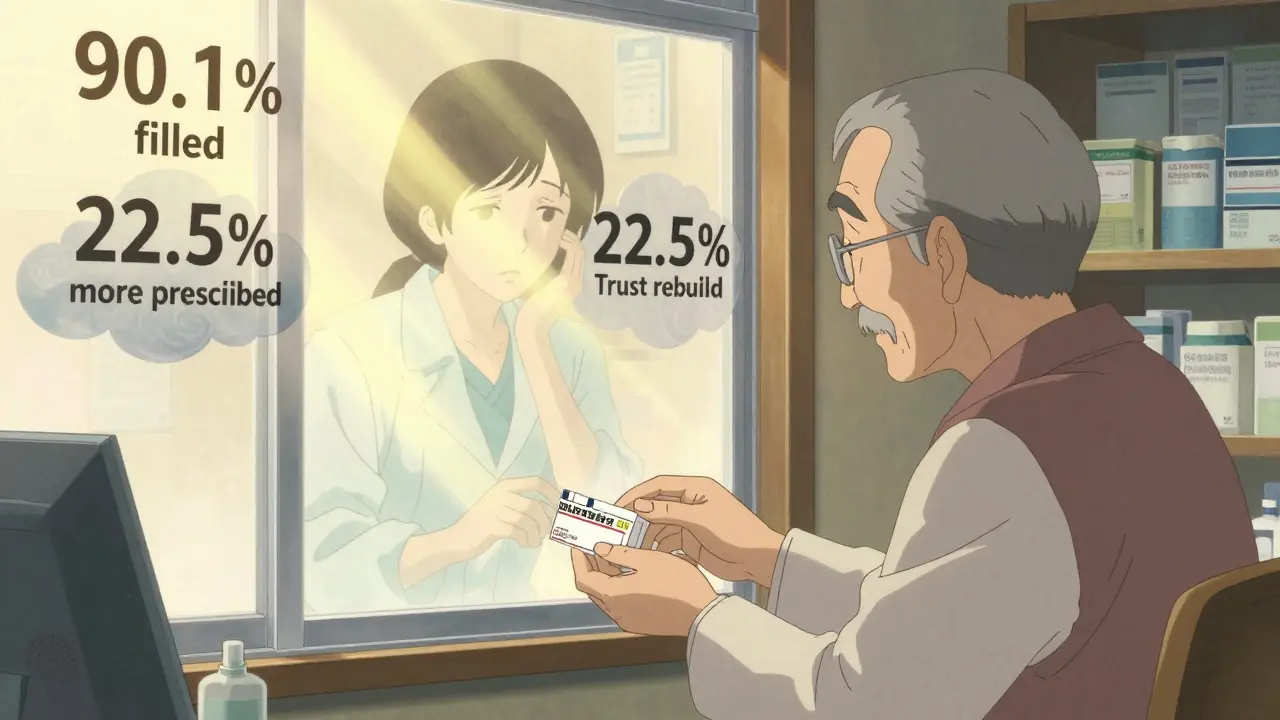
What needs to change?
It’s not about forcing doctors to prescribe generics. It’s about giving them the tools to feel confident.- Medical schools need to teach bioequivalence properly - not as an afterthought, but as core pharmacology.
- Continuing education should include case studies, not just slides. Show what happens when a patient switches - and when they don’t.
- Real-time data dashboards could show doctors how their patients are doing on generics compared to brands. No theory. Just results.
- Standardized naming - the AMA’s 2024 push to rename generics with easier-to-say names - helps. Doctors don’t want to say “sertraline hydrochloride” when they mean Zoloft. Clear names reduce confusion.
- Peer educators - doctors who’ve made the switch - are the most powerful tool. Their stories carry weight.
The cost of hesitation
Generics save the U.S. healthcare system over $300 billion a year. Globally, the market is worth $528 billion and growing at 7.3% annually. But if doctors keep doubting them, those savings stay locked away. Worse - every time a doctor hesitates, they reinforce a patient’s fear. That fear doesn’t just cost money. It costs trust. The solution isn’t more ads. It’s better education. Better data. Better conversations. Doctors aren’t resisting generics because they’re stubborn. They’re resisting because they’ve never been shown the proof - clearly, simply, and personally.What patients can do
If you’re on a generic drug and feel fine - tell your doctor. If you’re worried about switching - ask for data. Say: “Can you show me how this generic compares to the brand?” Most won’t have it ready. But if enough patients ask, the system will change. Change doesn’t come from mandates. It comes from questions. From curiosity. From doctors who finally see the evidence - and realize they’ve been wrong.Do doctors really think generic drugs are less effective?
Yes, a significant number do. Studies show that between 25% and 28% of physicians still believe generics are less effective than brand-name drugs, even though regulatory agencies confirm they are bioequivalent. This belief is stronger among older, male, and specialist doctors, particularly those treating conditions like epilepsy, thyroid disease, or heart rhythm disorders.
Why do some doctors avoid prescribing generics?
Many doctors avoid generics because they lack clear, real-world evidence showing how they perform in actual patients. They worry about small differences in absorption, especially with narrow-therapeutic-index drugs like warfarin or levothyroxine. Others cite poor continuing education, lack of time during appointments, and pressure from patients who distrust generics.
Are generic drugs regulated differently than brand-name drugs?
No - in the U.S., the FDA requires generics to have the same active ingredient, strength, dosage form, and route of administration as the brand. They must also prove bioequivalence (80-125% absorption range). In Europe, the EMA uses a tighter range (90-111%), which some experts believe contributes to higher trust in generics there. Both agencies require identical manufacturing standards.
Can switching to a generic cause side effects?
For most people, no. But in rare cases with drugs that have a narrow therapeutic index - like levothyroxine, phenytoin, or warfarin - even small differences in absorption can lead to noticeable changes. These aren’t caused by the generic being unsafe, but by how sensitive the body is to precise levels. That’s why some doctors prefer to stick with one version once a patient is stable.
How can doctors be convinced to prescribe more generics?
The most effective method is showing them real patient outcomes - not just regulatory data. Workshops that present before-and-after clinical results from patients who switched to generics have been shown to increase prescribing rates by over 20%. Peer-led education, where trusted colleagues share their success stories, works better than external training. Access to live dashboards of real-world effectiveness data is also proving powerful.
Do patients influence whether doctors prescribe generics?
Absolutely. About 68% of patients form their opinions about generics based on what their doctor says. If a doctor expresses doubt - even subtly - patients often refuse the cheaper option. In rural areas, 42% of patients stopped taking their meds after being switched to a generic, not because of side effects, but because they lost trust in their provider’s judgment.
Is there a financial incentive for doctors to avoid generics?
No. Doctors don’t earn more by prescribing brand-name drugs. In fact, many health systems encourage generic prescribing because it reduces costs. The barrier isn’t money - it’s perception. Doctors avoid generics because they’re unsure about safety and efficacy, not because they’re being paid to push brands.
What’s being done to improve doctor confidence in generics?
The FDA’s GDUFA III program now requires more post-market data on generics. Pilot programs at hospitals like Johns Hopkins are sharing real patient outcomes with prescribers - and seeing prescribing rates rise. Medical schools are slowly adding more pharmacology training on generics. The American Medical Association is also pushing for simpler, pronounceable generic names to reduce confusion. These steps are small, but they’re moving the needle.
Palesa Makuru
January 4, 2026 AT 10:52Okay but let’s be real - if your doctor still thinks generics are ‘less effective,’ they probably haven’t opened a textbook since 2008. I’m a pharmacist in Cape Town and I’ve seen patients on generics for years - same results, 1/10th the cost. The real issue? Doctors get lazy. They don’t wanna explain bioequivalence to a patient who’s already convinced the generic is ‘poison.’
Lori Jackson
January 6, 2026 AT 09:45Let’s not romanticize the FDA’s 80–125% bioequivalence window - that’s a 45% variance in absorption. That’s not science, that’s a gamble with someone’s neurological stability. If you’re prescribing levothyroxine or warfarin and calling it ‘equivalent,’ you’re not a clinician - you’re a cost-center administrator with a medical license.
veronica guillen giles
January 7, 2026 AT 21:54Wow. So the solution is… more jargon? More slides? More ‘studies’? 😒 How about this: doctors who’ve seen real patients switch successfully - let them talk. No PowerPoint. No FDA charts. Just ‘Hey, my patient on generic levo was stable for 18 months, no seizures, no crashes.’ That’s how humans change minds. Not lectures. Stories.
JUNE OHM
January 9, 2026 AT 07:55THEY’RE HIDING SOMETHING!!! 🚨 The FDA, Big Pharma, WHO - they’re all in cahoots to make us take ‘generic poison’ so the elites can control our minds with chemtrails in the water supply. I switched to organic turmeric and now my thyroid is ‘balanced’ (according to my crystal healer). Also, why do all the doctors who trust generics have ‘.edu’ emails? SUS. 🤫💊 #GenericGate
Shanahan Crowell
January 9, 2026 AT 21:23Listen - I used to be the guy who only prescribed brand names. Then I started tracking my patients on generics - no more ER visits, no more complaints, no more ‘I feel weird’ after the switch. I’ve got 37 patients on generic levothyroxine. All fine. All stable. All saving $80/month. If you’re still scared - look at the data. Not the fear. The data.
Kerry Howarth
January 10, 2026 AT 14:48Doctors don’t distrust generics because they’re ignorant. They distrust them because they’ve seen patients destabilize after a switch. The data says ‘equivalent.’ The clinic says ‘changed.’ There’s a gap. Fix the gap with real-world outcomes - not theory.
Joy F
January 11, 2026 AT 21:38Let’s deconstruct the epistemological crisis here. The doctor-patient dyad is a hermeneutic loop of inherited trauma - where the physician’s hesitation becomes the patient’s existential dread. The generic isn’t the problem. It’s the ontological insecurity of a medical system that commodifies trust. We’re not talking about pills. We’re talking about the collapse of epistemic authority in late-stage capitalism. And yes - I’ve seen three patients cry when their Zoloft became sertraline. That’s not pharmacology. That’s grief.
Haley Parizo
January 12, 2026 AT 11:57This isn’t about drugs. It’s about control. Who gets to decide what’s safe? The FDA? The pharmacist? Or the doctor who’s been told for 20 years that ‘brand = better’? We’re not talking bioequivalence - we’re talking cultural programming. And until we rewire medical education to question authority - not just memorize it - nothing changes.
Brittany Wallace
January 13, 2026 AT 02:32I’m a nurse in rural Ohio. I’ve watched patients cry because their doctor wouldn’t let them stay on the brand. They said, ‘I don’t know why, but I feel like I’m dying.’ It wasn’t the drug. It was the fear. We need doctors who can sit with that fear - not just write a script. Maybe if we taught empathy in med school, not just pharmacokinetics, we’d be better off.
Liam Tanner
January 13, 2026 AT 13:36My dad’s a cardiologist. He switched to generics after seeing his own brother’s BP drop too low on a new batch. He still won’t prescribe them for atrial fibrillation patients. I get it. But he also refuses to look at the Johns Hopkins dashboard showing 98% success rates. Stubborn? Maybe. But he’s seen the worst-case scenario. We need more than data. We need compassion.
erica yabut
January 13, 2026 AT 17:12Generic drugs are the corporate dystopia’s finest trick. You think you’re saving money? You’re just becoming a lab rat in Big Pharma’s ‘cost-cutting experiment.’ I read a paper that said the inactive ingredients in generics can contain glyphosate residues. And the FDA? They don’t test for that. Because why would they? They’re paid by the same companies that make the brand names. Wake up, sheeple. 💊👁️
Vincent Sunio
January 14, 2026 AT 20:20It is not logically coherent to assert that a 45% variance in bioavailability constitutes therapeutic equivalence. The FDA standard is scientifically indefensible. European regulators, by contrast, enforce a 21% tighter margin - and yet, American physicians are expected to accept inferior standards as ‘equivalent.’ This is not evidence-based medicine. It is regulatory capitulation disguised as cost-efficiency.
Tiffany Channell
January 15, 2026 AT 22:14Let’s cut through the noise. The only reason doctors hesitate is because they’re terrified of being sued. One patient has a seizure after switching to generic phenytoin - and suddenly, they’re the villain. No one ever gets sued for prescribing the brand. So they play it safe. Not because they’re wrong. Because the system punishes risk - even when the risk is statistically negligible.