When a doctor writes a prescription, they’re not just choosing a drug-they’re choosing a cost, a habit, and a belief. For most patients, the difference between a brand-name pill and its generic version seems simple: one costs $350, the other $4. But behind that price gap is a complex web of trust, perception, and clinical reality. And doctors, despite being the gatekeepers of prescriptions, aren’t always the ones pushing generics-even when the science says they should.
Generics Are Just as Effective. So Why Don’t More Doctors Prescribe Them?
By 2023, generics made up 90% of all prescriptions filled in the U.S. But only 72% of new prescriptions were written as generics. That gap tells you something important: doctors aren’t starting patients on generics as often as they could. Why?
It’s not because generics are less effective. The FDA requires them to deliver the exact same amount of active ingredient into the bloodstream as the brand-name drug, within a tight 80-125% range. That’s not a suggestion-it’s a legal standard. The same manufacturing rules apply. The same inspections. The same quality controls. A generic lisinopril isn’t a “weaker” version of Zestril. It’s the same molecule, made by a different company.
Yet studies show many physicians still default to brand names. A 2015 review found that primary care doctors in Saudi Arabia prescribed generics 47% of the time, while hospital-based physicians did so only 31% of the time. In Greece, half of doctors said generics were “high or very high” quality-but only 25% actually prescribed them. Something deeper than knowledge is at play.
It’s Not About Cost-It’s About Control
Here’s the twist: doctors aren’t paying for these drugs. Patients are. So why would a doctor care about cost? Because cost affects adherence. And adherence affects hospital visits, complications, and long-term outcomes.
Research from the American College of Physicians shows that patients on generics are 6% more likely to stick with their medication. That might sound small, but it translates to a 2.2% drop in hospitalizations for chronic conditions like high blood pressure or diabetes. That’s not just a savings-it’s lives saved.
But doctors aren’t always thinking about adherence. They’re thinking about control. A patient walks in, says, “My cousin took the generic and felt awful.” Or, “My insurance covered the brand last month, so I want that one.” The doctor, caught between evidence and emotion, often gives in-not because they doubt the science, but because they don’t want to fight.
One internist in Texas told a Reddit thread: “I’ve had patients insist on brand-name lisinopril costing $350/month when the generic is $4 at Walmart. I show them the data. They still refuse.”
Appearance Matters More Than You Think
Patients don’t just judge drugs by their active ingredients. They judge them by their shape, color, and size. A generic pill might look completely different from the brand-name one they’ve been taking for years. That’s not a flaw-it’s how manufacturing works. But the brain doesn’t care about bioequivalence studies. It cares about familiarity.
The FDA’s “Look Alike Sound Alike” program, launched in 2018, cut patient confusion by 37%. That’s because they started pushing manufacturers to avoid designs that mimic other drugs. But it’s still a problem. A 2015 FDA study found patients had “mixed perceptions of efficacy, safety, and quality” simply because the pill looked different.
One elderly woman in Bristol switched from her brand-name blood pressure pill to the generic. She noticed the color changed from blue to white. She stopped taking it. “It just didn’t feel right,” she told her pharmacist. No one told her the active ingredient was identical. No one explained that the change was normal.
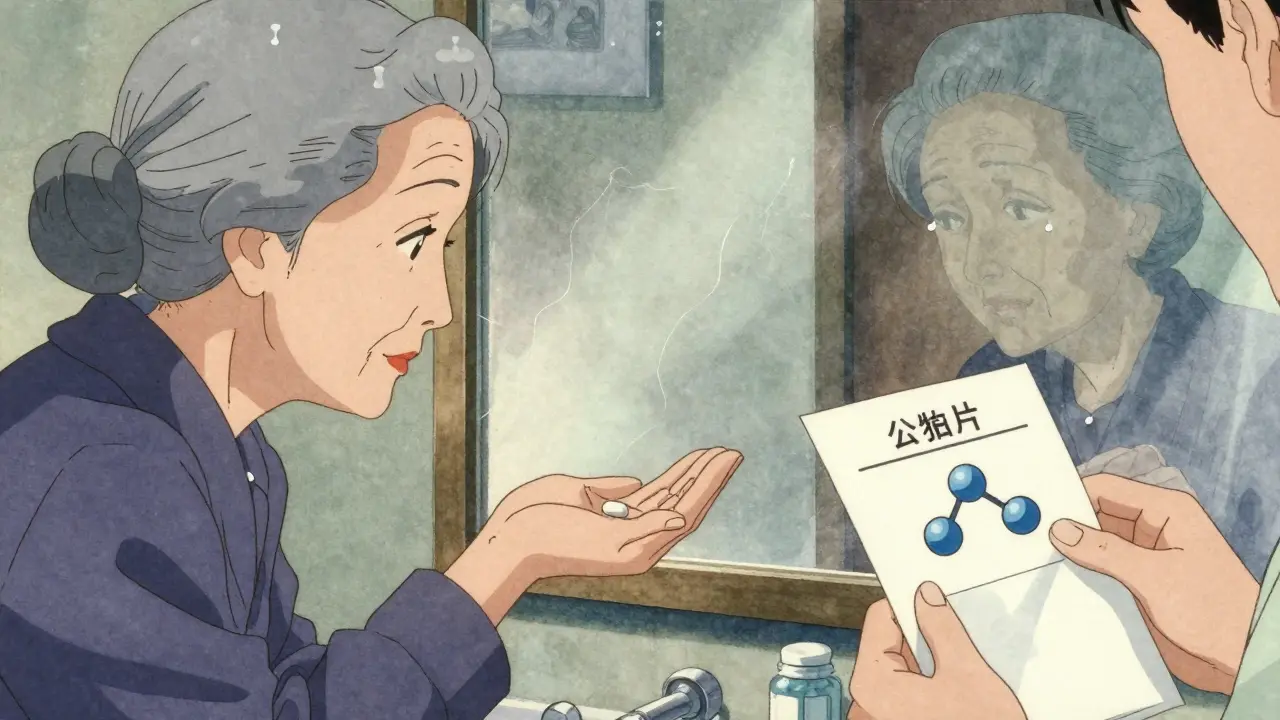
Doctors Are Learning-But Slowly
Education is changing. In 2015, only 29% of internal medicine residency programs in the U.S. taught generic prescribing. By 2026, that number jumped to 68%. The American Medical Association found that doctors who completed FDA-sponsored training on generics increased their prescribing rates by 23% within six months.
But training alone isn’t enough. The real barrier is habit. Many doctors were trained in an era when generics were seen as “second-tier.” That mindset sticks. Even when they know better, they default to what’s familiar.
And then there’s the legal layer. In 49 states, pharmacists can switch a brand-name drug for a generic without asking the doctor-unless the doctor specifically writes “Dispense as Written.” That means a patient might get a different pill than they expected, without ever knowing why. No warning. No explanation.
Where Generics Still Raise Red Flags
Not all drugs are created equal. For medications with a narrow therapeutic index-like warfarin, levothyroxine, or phenytoin-even tiny differences in absorption can be dangerous. The FDA keeps a list of 15 such drugs where substitution requires extra care. For these, doctors often stick with brand names, not because they distrust generics, but because they’re being cautious.
Even then, the data doesn’t support fear. A 2021 study tracking over 136,000 older adults switching from brand-name blood pressure drugs to generics found a small uptick in ER visits. But researchers couldn’t prove the generics caused it. They suspected other factors: patients forgetting doses, changing pharmacies, or stopping meds altogether after the switch.
Meanwhile, Medicare data shows patients on generics have 12.7% higher adherence rates than those on brand names. That’s not a fluke. That’s a pattern.
What Patients Need to Hear
The biggest gap isn’t between brand and generic. It’s between doctor and patient.
When a doctor says, “I’m switching you to the generic,” they’re often not saying: “This is the exact same drug.” They’re saying: “This will cost less.” And that’s not enough.
Patients need to hear: “The active ingredient is identical. The FDA requires it. You’ll get the same effect. The only difference is the price.”
Pharmacists can help. The FDA’s Dr. Sarah Ibrahim says patients are more likely to stop taking generics when they experience a change-without explanation. That’s why pharmacist counseling matters. A simple conversation before the switch can prevent 40% of non-adherence.
And patients need to know: if you’ve been on a brand-name drug for years, switching to a generic doesn’t mean you’re being “downgraded.” It means you’re being smart.
The Future Is Generic
The global generic market is projected to hit $595 billion by 2028. Biosimilars-generic versions of complex biologic drugs-are already gaining ground. In 2027, they could make up 15% of biologic prescriptions.
But adoption won’t happen by itself. It needs doctors who are confident enough to lead with evidence. It needs patients who trust the science. And it needs systems that don’t let confusion slip through the cracks.
Right now, the U.S. could save $17.3 billion a year in Medicare Part D costs if every new prescription started with a generic-instead of a brand name. That’s not a pipe dream. It’s math.
Doctors aren’t against generics. They’re just not always taught how to talk about them. And patients aren’t against generics-they’re just afraid of what they don’t understand.
The fix isn’t more regulations. It’s better conversations.

Jonathan Noe
February 10, 2026 AT 12:23Jim Johnson
February 10, 2026 AT 19:28Vamsi Krishna
February 10, 2026 AT 19:29Brad Ralph
February 12, 2026 AT 12:36Suzette Smith
February 14, 2026 AT 08:20Autumn Frankart
February 15, 2026 AT 19:31Stephon Devereux
February 16, 2026 AT 01:10steve sunio
February 16, 2026 AT 13:21athmaja biju
February 18, 2026 AT 02:09Robert Petersen
February 19, 2026 AT 01:29Reggie McIntyre
February 19, 2026 AT 11:03Carla McKinney
February 19, 2026 AT 19:31