When you take two drugs that each have a razor-thin margin between helping you and harming you, and then try to swap them out for cheaper generic versions, things can go wrong-fast. This isn’t theoretical. It’s happening in hospitals, pharmacies, and living rooms across the U.S. every day. These are NTI drugs-narrow therapeutic index medications-where even tiny changes in blood levels can cause serious harm or treatment failure. Now imagine combining two of them. That’s where the real danger lies, and where the generic drug system is failing patients.
What Makes a Drug an NTI Drug?
An NTI drug has almost no room for error. The difference between the dose that works and the dose that kills is smaller than most people realize. For example, warfarin-a blood thinner-has a therapeutic window so narrow that a 10% change in blood concentration can mean the difference between preventing a stroke and causing a bleed. Same with levothyroxine for thyroid disease, lithium for bipolar disorder, or phenytoin for seizures. The FDA defines these drugs by five key traits: minimal separation between safe and toxic levels, high risk of life-threatening side effects from small changes, need for frequent blood monitoring, low variability in how the body processes them, and frequent small dose tweaks by doctors.
These aren’t obscure drugs. They’re some of the most commonly prescribed in chronic care. Over 30 NTI drugs are on the market today. And while single-agent versions of most have generic equivalents, the moment you combine two NTI drugs into one pill or regimen, the rules change completely.
Why Combination NTI Drugs Are a Perfect Storm
Combination therapy isn’t new. In tuberculosis, doctors have long used isoniazid (an NTI drug) with rifampin to prevent resistance. In cancer, methotrexate is paired with other agents to kill tumors more effectively. The idea is simple: hit the disease from multiple angles. But when both drugs are NTI drugs, the math gets dangerous.
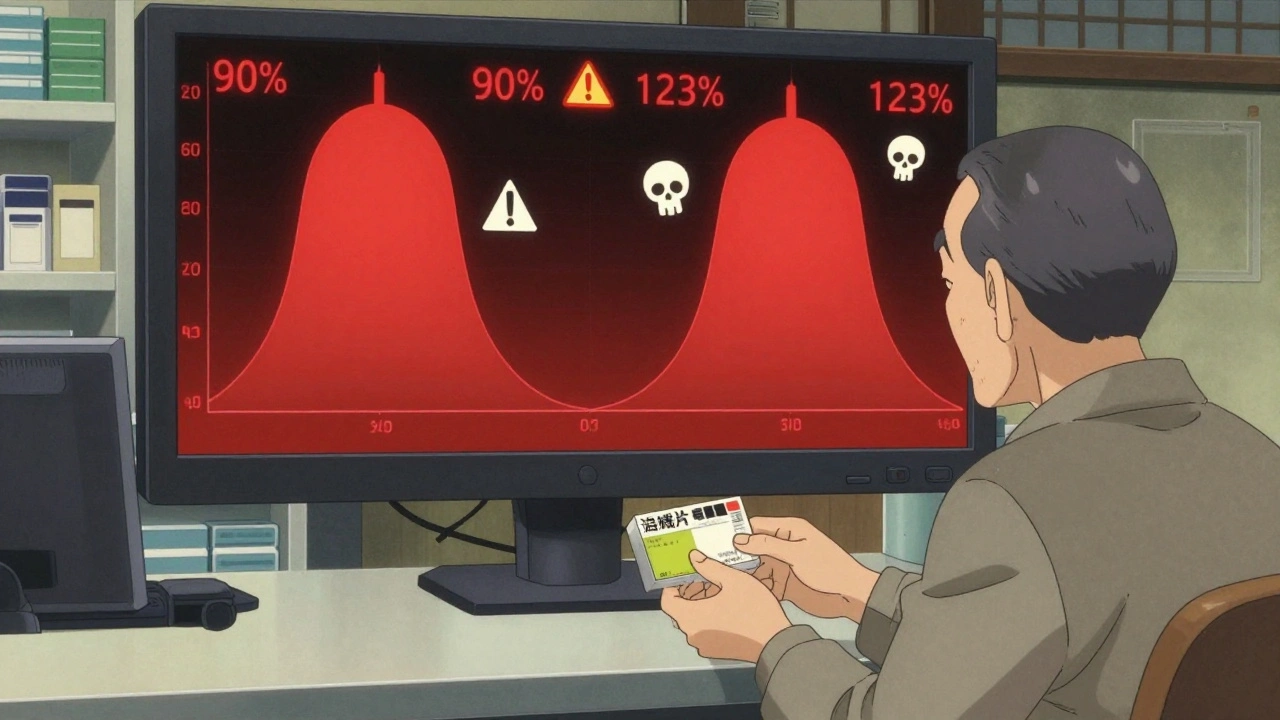
Let’s say Drug A has a bioequivalence window of 90%-111% (the FDA’s strict standard for NTI drugs). Drug B also has the same window. When taken together, the total variation isn’t just 90%-111%. It’s compounded. The combined effect could swing anywhere from 81% to 123% of the intended dose. That’s not a small fluctuation-that’s a clinical emergency waiting to happen.
Real-world data backs this up. A 2020 JAMA Internal Medicine study found patients on combination therapies containing even one NTI drug had a 27% higher chance of adverse events after switching to generics-compared to just 8% for non-NTI combinations. When both drugs are NTI, the risk skyrockets. One patient on Reddit described their INR (a blood clotting measure) spiking from 2.5 to 6.8 after switching to generic warfarin while on another NTI drug. They ended up hospitalized. That’s not an outlier. It’s a pattern.
The Regulatory Wall
The FDA set strict bioequivalence rules for NTI drugs back in 2014 and updated them again in 2022. For a single NTI drug, generic manufacturers must prove their product stays within 90.00%-111.11% of the brand’s Cmax (peak concentration) and 90.00%-112.00% for AUC (total exposure). That’s tighter than the standard 80%-125% used for most generics. But for combination NTI drugs? There’s no approved pathway.
As of October 2023, the FDA Orange Book lists zero approved fixed-dose combinations of two NTI drugs in the U.S. Not one. While generic warfarin, digoxin, and lithium are widely available, no company has successfully brought a combo like warfarin + amiodarone or lithium + phenytoin to market. Why? Because the science doesn’t exist yet. Current testing methods can’t reliably measure how two NTI drugs interact in the body when each has its own narrow window.
The FDA’s 2023 draft guidance tried to address this, proposing even tighter standards for combinations: 90.00%-107.69% for Cmax and 90.00%-110.00% for AUC. But that’s still a 17.69% swing for one drug and 10% for the other. When multiplied, it’s a 27.69% total variation. That’s still too wide for safety.
What Happens When There’s No Generic?
Patients are stuck paying brand prices for combo NTI regimens. A single pill of a brand-name NTI combination can cost $500-$1,200 per month. That’s more than most insurance plans will cover without prior authorization. Many patients are forced to take two separate pills-each potentially generic, but not tested together. That’s a nightmare for adherence. One pill is easy. Two pills, taken at different times, with different dosing schedules? Many patients miss doses or take them wrong.
And then there’s the monitoring cost. Patients on NTI combinations need frequent blood tests-sometimes weekly at first. The annual cost for therapeutic drug monitoring runs $1,200 to $2,500 per person. Compare that to $400-$800 for non-NTI combinations. That’s not just a financial burden-it’s a logistical one. Many rural patients can’t get to labs often enough. Pharmacists are overwhelmed. A 2023 ASHP survey found 78% of pharmacists had seen treatment failure after generic switches in NTI combos. Over 40% reported serious adverse events, including hospitalizations.
Are There Any Success Stories?
Yes-but they’re rare. In Europe, some combination products containing levothyroxine and selenium have been used safely since 2015. But these are exceptions. Levothyroxine, while an NTI drug, has relatively low variability in absorption when taken on an empty stomach. Selenium isn’t an NTI drug. So the risk profile is different. Even then, European regulators require strict patient education and monitoring.
On Reddit, one user wrote: “My levothyroxine and selenium generics have worked fine for two years.” But that’s one person. FDA data shows less than 15% of combination NTI scenarios have stable outcomes with generics. The rest? Unpredictable. That’s why most hospital systems-87% of them-block automatic substitution for NTI combinations. Community pharmacies? They’re more likely to switch, often without telling the prescriber.
The Human Cost
A 2022 Drugs.com survey of 1,247 patients on NTI combinations found 63.4% experienced side effects after switching to generics. That’s more than six in ten. Symptoms included dizziness, irregular heartbeat, confusion, bleeding, or seizures. Many didn’t connect the dots until they went back to the brand. One woman wrote: “I thought my fatigue was from aging. Turns out, my pharmacy switched my warfarin and lithium generics. My INR was off the charts.”
Doctors are caught in the middle. They know the risks. But they also know their patients can’t afford the brand. So they do what they can: write “Dispense as Written” on prescriptions, order more lab tests, spend extra time counseling. But it’s not scalable. Only 12 of 50 major U.S. academic hospitals have specialized NTI combination clinics. Most patients are left to navigate this alone.

What’s Next?
The FDA is exploring a pilot program for “precision bioequivalence” testing using pharmacometric modeling-essentially computer simulations that predict how drugs interact in real patients. It’s promising. But it’s years away from being routine. Meanwhile, generic manufacturers like Teva and Sandoz are pushing for faster approvals, citing advances in manufacturing precision. But even the best manufacturing can’t fix a flawed scientific standard.
Some experts, like Dr. Robert Temple (retired FDA), believe technology will eventually catch up. Others, like Dr. Lewis Nelson of NYU, say the fundamental problem is unsolvable with current methods: “Combining two narrow-window drugs creates a system too sensitive for generic substitution to be safe.”
The truth? We’re in a gap. A massive one. Patients need affordable options. But safety can’t be sacrificed. Until we have better science, better regulations, and better monitoring tools, combination NTI drugs will remain a luxury only the wealthy can afford safely.
What Can You Do?
- If you’re on a combination of NTI drugs, ask your pharmacist: “Is this a brand or generic? Are both components generic?”
- Never let your pharmacy switch your meds without telling you. Demand to be notified.
- Keep a log of your symptoms and lab results. If your INR, lithium level, or seizure frequency changes after a switch, report it immediately.
- Ask your doctor to write “Dispense as Written” on your prescription. It’s legal, and it can save your life.
- Advocate. Tell your state pharmacy board and elected officials that NTI combination drugs need special protection.
What are NTI drugs?
NTI drugs, or narrow therapeutic index drugs, have a very small difference between the dose that works and the dose that causes harm. Examples include warfarin, lithium, levothyroxine, phenytoin, and digoxin. Even small changes in blood levels can lead to serious side effects or treatment failure.
Why are combination NTI drugs risky with generics?
When two NTI drugs are combined, the small variations allowed in each generic drug multiply. For example, if each drug can vary by up to 11%, together they could vary by over 20%. That’s enough to push blood levels into toxic or ineffective ranges, leading to hospitalizations or treatment failure.
Are there any generic combination NTI drugs available in the U.S.?
No. As of 2023, the FDA has approved zero fixed-dose combination products containing two NTI drugs. While single-agent NTI generics are common, no manufacturer has met the bioequivalence standards required for combinations of two NTI drugs.
How do I know if I’m on an NTI drug combination?
Check your medication list against the FDA’s NTI drug list. Common ones include warfarin, lithium, levothyroxine, phenytoin, carbamazepine, and digoxin. If you’re taking two of these together, you’re on a combination NTI regimen. Ask your pharmacist or doctor to confirm.
What should I do if my pharmacy switches my NTI meds?
Contact your prescriber immediately. Don’t wait for symptoms. Request a blood test to check levels (like INR for warfarin or lithium levels). Ask for your original brand to be reinstated. Always ask for a written prescription that says “Dispense as Written” to prevent future switches.
Why don’t generic companies make these combinations?
Because the science doesn’t currently support it. The FDA’s bioequivalence standards for NTI drugs are already stricter than normal, and combining two NTI drugs makes proving equivalence nearly impossible with today’s testing methods. The cost and risk of failure outweigh the potential profit.
Can I trust European NTI combination generics?
Some, like levothyroxine + selenium combinations, have been used safely in Europe with strict monitoring. But these are exceptions. The drugs involved have lower variability, and European regulators require more intensive follow-up. Don’t assume what works there will work the same way here without the same safeguards.
Final Thoughts
This isn’t about brand vs. generic. It’s about safety vs. savings. We can’t afford to let cost-cutting override clinical judgment when lives are on the line. Combination NTI drugs are a blind spot in our drug system. Until regulators, manufacturers, and prescribers fix the science and the policy, patients will keep paying the price-in hospital bills, in lost time, and in fear.

Abhi Yadav
December 3, 2025 AT 15:12So we just let people die because Big Pharma wants profits? 😔
Real talk: if your life depends on a pill and the system lets a cheaper version kill you, that’s not capitalism, that’s murder.
And don’t give me that ‘science isn’t ready’ crap-people are already dying.
Why do we wait for bodies before we act?
Just sayin’.
Julia Jakob
December 4, 2025 AT 16:11my pharmacy switched my warfarin + lithium combo last year and i didn’t even notice til i started seeing stars and my heart felt like it was tryna escape my chest
took me 3 weeks to get my doc to listen
now they just write DAW on everything
but why does it have to be this hard??
Nancy M
December 4, 2025 AT 16:21There’s a profound irony here: we demand affordable healthcare, yet we resist the very systems that could make it safe.
The FDA’s caution isn’t bureaucracy-it’s a moral stance.
But the absence of regulation is not freedom; it’s negligence.
Patients deserve both access and safety.
And yet, the system forces us to choose.
That’s not progress.
That’s failure dressed in policy.
Maybe the answer isn’t more generics-it’s better science, better oversight, and better accountability.
Until then, we’re gambling with lives.
And that’s not a risk any society should take.
gladys morante
December 4, 2025 AT 17:17I’ve been on levothyroxine and carbamazepine for 12 years. My levels are stable. But my pharmacy switched the generics last fall. I didn’t realize until I couldn’t get out of bed for three days. They told me it was ‘bioequivalent.’ I told them I was dying. They didn’t care. Now I pay out of pocket. No one wins.
Precious Angel
December 6, 2025 AT 12:27Let me be brutally honest-this isn’t about science, it’s about greed.
The FDA is a puppet of Big Pharma.
They let generic manufacturers get away with murder because they don’t want to slow down the profit machine.
And you think this is an accident? NO.
It’s systematic.
Every time someone gets hospitalized because their lithium level spiked after a switch, it’s a corporate win.
Insurance companies save $30 a month.
Patients lose their kidneys, their minds, their lives.
And the FDA? They publish guidelines that look good on paper while real people bleed out in ERs.
This isn’t a gap.
This is a massacre.
And we’re all just scrolling past it.
Wake up.
David Ross
December 7, 2025 AT 06:20As an American veteran who’s seen the VA’s drug protocols, I can tell you: this is why we can’t have nice things.
Generic substitution for NTI combos is a national disgrace.
It’s not just dangerous-it’s un-American.
Our founding principle was liberty, not cost-cutting at the expense of life.
And yet here we are: letting Chinese manufacturers test half-baked pills on our grandparents.
Enough.
Fix the system or ban generics for NTI drugs entirely.
There is no middle ground when people are dying.
Sophia Lyateva
December 8, 2025 AT 13:37they’re putting fluoride in the water and switching our meds to make us docile
you think this is a mistake? no
it’s the new eugenics
they want us weak, confused, and dependent
the same people who made the 2023 draft guidance also pushed the covid boosters
connect the dots
they don’t care if you live or die
they just want control
AARON HERNANDEZ ZAVALA
December 9, 2025 AT 06:39I’ve worked in rural pharmacies for 15 years.
People don’t have time or money for weekly blood draws.
They take their meds, hope for the best, and pray the pharmacist doesn’t switch anything.
Most don’t even know what NTI means.
And when they get sick, they blame themselves.
It’s heartbreaking.
We need better education, not just stricter rules.
Doctors should be required to explain this to patients in plain language.
And pharmacists? They need to be forced to ask before switching.
It’s not about stopping generics.
It’s about stopping carelessness.
Robert Asel
December 10, 2025 AT 06:43There is a fundamental misunderstanding in this discourse: bioequivalence is not a suggestion-it is a regulatory standard grounded in pharmacokinetic science.
Combination NTI drugs present a multivariate problem that current in vivo models cannot resolve with acceptable confidence.
It is not a failure of industry-it is a failure of methodology.
Until we develop validated in silico models capable of simulating drug-drug interactions under population variability, any approval would be scientifically indefensible.
Patients deserve safety, not expedience.
And yet, the public demands cheaper drugs without understanding the trade-offs.
Blaming regulators is easy.
Fixing the science is hard.
And that is the real challenge.
Ben Wood
December 11, 2025 AT 10:23Let’s be real: if you’re on two NTI drugs, you’re already in the 1% of patients who need elite-level medical care.
So why are you even on generics?
You should be on brand-name, monitored weekly, and under a specialist’s care.
Stop trying to save $40 a month when your life is on the line.
This isn’t about affordability.
This is about people refusing to accept that some things are too dangerous to cut corners on.
Stop blaming the system.
Start taking responsibility for your own health.
And if you can’t afford it?
Then get a better job.
Or move to a country that actually cares about patient safety.
Because in America, we don’t get to have both cheap and safe.
Choose.